Assessment of physical activities and physical fitness ppt. Do metals more readily gain or lose electrons.
6 5 Metals Chemistry Libretexts
In cannot be dropped because the user owns some object.

. The reason is that they have a few extra electrons in outer shells above an inner core. Generally elements that find it hard to lose electrons do so for reasons that also make it easy to gain electrons high effective nuclear charge and low number of energy levels. Answer 1 of 5.
If the element is more electronegative the tendency of it to keep the electrons in it is more. 247 help from Expert Tutors on 140 subjects. Only a few metals gold and platinum are two examples appear in nature in metallic form.
They tend to lose electrons. The outer electrons of most metal atoms tend to be weakly held to the atomic nucleus. What is an alloy.
Magnetic resonance spectroscopy ppt. 15 - Sulfuric acid H2SO4 loses two protons to form. 15 - Why is it so easy for a magnesium atom to lose two.
What is a native metal. Do metals more readily gain or lose electrons. The exceptions to this generality are the inert gases which find it both hard to lose electrons because of high effective nuclear charge and hard to gain electrons because of a full outer energy level.
Loosing these outer electrons results in an ion. On the other hand halogens such as chlorine only need to gain one electron to form a full outer shell. Tacoma news tribune death notices 2021.
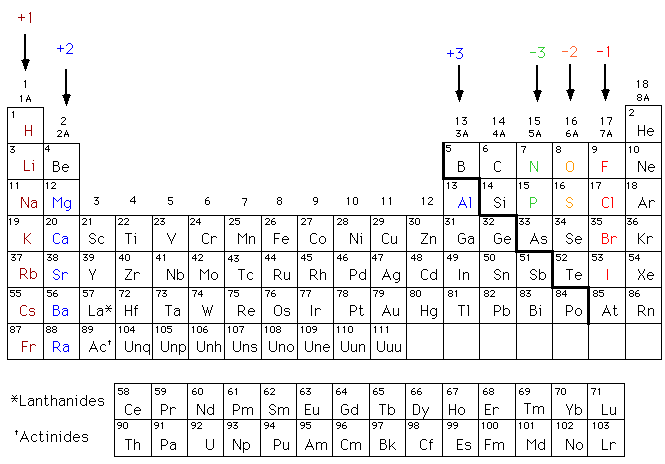
Elements that are metals tend to lose electrons and become positively charged ions called cations. Do metals more readily gain or lose electrons They easily loose. The ionization energy of metals is lower than the ionization energy necessary to take away electron from an atom.
Metals that are located in column 1A of. Metals that are located in column 1A of the periodic table form ions by losing one electron. It takes some energy yes but not much.
Benjamin moore fresh start primer instructions. Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions. Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions.
Alkaline metals for example would find it much easier to lose electrons than gain electrons so they are not very electronegative. 15 - Why doesnt the neon atom tend to lose or gain any. Metals tend to give away electrons to form positively charged ions while non metals tend to gain electrons to become negatively charged.
15 - Which should be more difficult to pull apart. Full access to over 1 million Textbook Solutions. 15 - Why does an atom with many valence electrons tend.
A mixture composed of two or more metallic elements Two or more metals bonded to each other by metallic bonds. Do metals gain or lose electrons when bonding. Get more out of your subscription Access to over 100 million course-specific study resources.
Wrap text in button bootstrap. Consequently these electrons are easily dislodged leaving behind positively charged metal ions. Elements that are metals tend to lose electrons and become positively charged ions called cations.
Its very easy for metals to lose electrons in general. This is much easier than losing seven electrons instead. Do metals gain or lose electrons when bonding North York ON M6A 2T9.

Be Existence Study Hard Now Learning About Ions Ionic Study Hard Study Time Study

Periodic Table Worksheet Answer Key Chemistry Worksheets Periodic Table Atomic Structure

0 Comments